Access, Stabilization, and Closure System
Apica Cardiovascular's Inventive Tool Eases Implementation of Heart Devices
If you're undergoing a serious operation like heart surgery, the last thing you want to hear is that the process is going to be complicated and intrusive. That's why across the medical industry, researchers are constantly looking for ways to make medicine and medicinal practices as unnoticeable – or as minimally invasive – as possible. A new technology out of the Ireland-based Apica Cardiovascular, Inc, looks to do just that, with a novel device that could dramatically change how doctors deliver medical devices to the heart.

Vinod Thourani, MD
When cardiac surgery is performed to insert a medical device, it is either done through an incision through the leg, known as transfemoral access, or an incision between the ribs of the chest, referred to as transapical access. The biggest concern for doctors performing the chest incision was figuring out how to make a hole in the apex of the heart and then close it with absolute certainty that it would remain sealed. Previously, the seal was closed using a method reminiscent to how a drawstring bag is closed. After a transapical access device had been inserted, it would create a circle of sutures around the area of operation. When the device was removed, it would draw these sutures together much like the closing of a drawstring bag, which could cause complications down the line as a result of the large amounts of scar tissue. Apica's new ASC, or Access, Stabilization, and Closure system directly addresses. The ASC system, a hollow, tube like structure, creates a stable port in the apex of the heart for delivery of therapeutic devices. The port can be easily sealed afterwards with a small closure cap, minimizing the scar tissue that forms. Also, not only is the device less invasive, but the procedure is much quicker to complete.

Thomas Vassiliades, MD, MBA
The ASC system was a product of a collaboration between two Georgia Tech engineers, Jorge Jimenez, PhD and Ajit Yoganathan, PhD, and two Emory Cardiac surgeons, Vinod Thourani, MD, and Thomas Vassiliades, MD, MBA. Apica CEO Jim Greene saw the original technology out of Georgia Tech, a coil-like device that could compress tissue, could have very useful applications as a medical instrument.
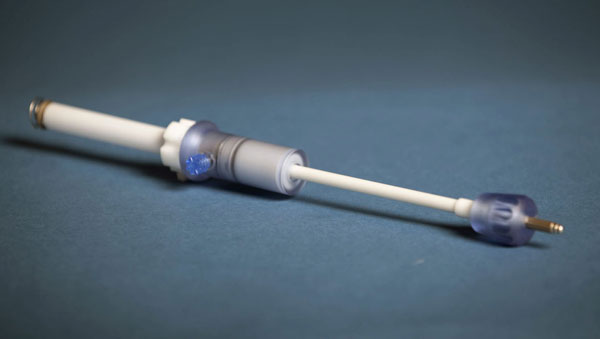
APICA Device
The device passed through clinical trials this January in several centers across Germany, leading to the result being published in The Thoracic and Cardiovascular Surgeon. Successfully meeting all safety and technical checkpoints during clinical trials also led to CE Mark approval, which means the ACS system is eligible for launch into specialist Transcatheter Aortic Valve Implantation (TAVI) centers across Europe. The ACS, also boasting a 100% technical success rate during trials and no complications in 90 day follow ups, already promises to be a major industry player, to the tune of what Apica R&D Director Brendan Cunniffe estimates could be upwards of 2 billion dollars. Already, Apica is targeting select German hospitals for device implementation. As if the fortunes for this young company weren't already exciting enough, Apica is already at work on the research and creation of other medical innovations.