Axumin
Building a Better Radiotracer
When Mark Goodman, Professor of Radiology & Imaging Sciences, first arrived at Emory in 1993, he initiated two research projects to develop imaging agents for unmet clinical needs. "One was to develop an imaging agent based upon an analog of cocaine," he recalled from his office in the Center for Systems Imaging. "The cocaine molecule is in a family of tropanes. We wanted to look at movement disorders and addiction, the effect of Parkinson's disease on certain areas of the brain and how that progresses."
Goodman and his team successfully synthesized their analog and demonstrated that it could measure the progression of Parkinson's Disease, but they opted not to patent the molecule for commercialization. Goodman's other project, a radiotracer made for cancer diagnostic imaging, was starting to show potential. He had been brought to Emory for his specialty in PET (positron emission tomography) radiochemistry and it seemed that he was on the precipice of a new discovery in the field. This discovery would become Axumin®, the PET imaging molecule now used to detect recurrent prostate cancer.

Mark Goodman, PhD
PET imaging works by introducing small amount of a biologically active molecule called a radiotracer into a patient's body as a scanner monitors the rate at which the molecules are taken up by different cells. Most PET scans are performed for cancer diagnosis using a tracer known as 2-FDG. This relies on the Warburg effect, the notion that cancer cells consume glucose at a higher rate than other cells. 2-FDG is a glucose analog, so practitioners can detect cancer cells by monitoring where it accumulates.
In the case of prostate cancer, however, complications arise. First, glucose eventually accumulates in the bladder, which is right above the prostate. This masks tracer radioactivity in the prostate and makes cancer detection difficult. Second, since most prostate cancer cases are slow to develop, prostate cancer cells tend to have a less pronounced rate of glycolysis. This contributes to the difficulty in distinguishing prostate cancer cells from other cell types
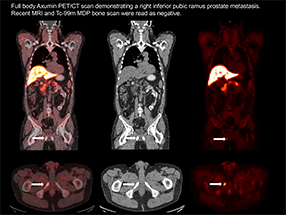
Axumin® Full Body Scan
"Axumin® has a very slow accumulation in the bladder," Goodman explained to me. Furthermore, it is transported into prostate cancer cells to such a high degree that it measures the rate of disease development in both low- and high-grade prostate cancer. As an added bonus, it is detectable in proximal and distal lymph nodes and bone, which can help guide biopsies and manage treatment
Upon his arrival at Emory, Goodman was approached by Nihon Medi-Physics (NMP) to sponsor the development of a 2-FDG alternative for use in SPECT (single-photon emission computed tomography) imaging – "It was very difficult. We did a lot of chemistry to attempt to develop an iodinated glucose analog labeled with the SPECT radioelement iodine-123 that behaved similar to 2-FDG. Unfortunately, the relatively small glucose molecule was not amenable to addition iodine. But, no one else was able to do it."

David Schuster, MD
NMP's research focus took an unexpected turn, and Axumin® was initially developed as an imaging molecule for a type of brain tumor known as a glioma. Goodman told NMP, "I said to them, "Listen, we're working on this amino acid and it looks really good in an animal model of glioma." So, we did some pilot studies in human glioma."
Goodman and David Schuster, MD, associate professor of radiology and imaging sciences at Emory, discovered that the radiotracer was especially good for looking at prostate cancer, because of its "low native urinary excretion," says Schuster. It proved superior to the conventional PET imaging agent—radioactive glucose—which is readily taken up by cancer cells but also appears in the urine, making detection more difficult since the prostate bed is close to the bladder.
Nihon Medi-Physics was impressed with the results. They agreed to fund further research on the condition that they could license the resultant technology. "After a year of work, they decided they were going to license it, so we submitted the patent," Goodman said. In addition to Axumin®, NMP funded research in Goodman's lab to evaluate several other molecules for use in both glioma and prostate cancer diagnosis. It was determined that Axumin® had the best properties.
The patent was submitted in 1995 and it was licensed by NMP in 2003. The company had been equally owned by two parent companies and in 2003, GE Healthcare bought one of them, giving them half ownership of NMP in the process. NMP sublicensed Axumin® to GE in 2008 and soon, Goodman ran into a problem. "GE was in competition with some other companies to bring to market a PET imaging agent for Alzheimer's disease," he told me. "GE put Axumin® aside because they figured the Alzheimer's market was in the billions, but the market for prostate cancer and glioma was only a fraction of that."
This would be only one of the many challenges involved in bringing Axumin® to market. Emory was unexpectedly able to only obtain patent protection on the PET imaging agent in the United States. The Japanese regulatory approval process for Axumin® has been lengthy, though development of the PET tracer for use in East Asia, where NMP had initially intended to market the drug, continues. "You would think the approval pathway would be smoother than for a drug, right?" Cale Lennon, the technology's case manager at the OTT, ironically noted. "It should be something, since it's only a radiotracer diagnostic imaging agent, that would take less time to obtain regulatory approval."
Two members of Axumin's team at GE Healthcare eventually left to found Blue Earth Diagnostics, a company dedicated to marketing Axumin®. "We were very pleased about that," Lennon recalls, "because a startup company was formed and funded with the specific aim to finish up regulatory approval and get our product to market." Thanks to an investment from Syncona, the venture capital arm of the famed Wellcome Trust, commercially available Axumin® finally became a reality in 2016.
"Once it was put into the hands of Blue Earth, they rapidly did everything to get it to market," Lennon furthered. "They used a lot of existing data that had been collected over the years in research studies for the regulatory submission, which was a really good strategy." Today, Axumin® is marketed by Blue Earth Diagnostics for recurrent prostate cancer – situations in which prostate cancer appears to have returned after an initial round of treatment.
The patent is set to expire sometime in 2020. "If we weren't academics," Goodman mused, "if we were a start-up company, we would look for ways to layer the patent and extend it. That wasn't our mindset though. We're talking 1995. I came here in 1993. In two years, we got a patent."
When the patent expires, Blue Earth will still be able to market Axumin®, but the possibility for generic alternatives will also open up. Goodman is unfazed by the competition – "There are some alternatives, other PET imaging agents, in clinical trials or going forth. Some may have advantage to Axumin®, but it's a matter of, is it incrementally better or is it significantly better? This is up to the U.S. Food and Drug Administration (FDA) and the insurance companies, not for me." "You can do good science, but when it comes to commercialization, you have to rely on a partner you have faith in," says Goodman. "It looks like this imaging agent will be out there for patient use. That feels great, it really does."
For the time being, Blue Earth is attempting to expand the indications for which Axumin® is approved. The brain uses a lot of glucose, so 2-FDG is ineffective for modeling its tumors. Axumin® uses compatible transport molecules on the blood-brain barrier to enter tumor cells, thus allowing for function-based imaging, rather than reliance on image contrast. Lennon confirmed that Blue Earth has submitted to the FDA a supplemental New Drug Application for the use of Axumin® in diagnosing glioma and metastatic brain cancer, as per Goodman's initial discoveries.
Goodman hopes that in time, Axumin® will also find approval for primary prostate cancer diagnoses. In theory, Axumin® would work as a diagnostic for other cancers. Glucose accumulation in the bladder obviously complicates the use of 2-FDG in bladder cancer diagnosis, and current diagnostic imaging technology insufficiently handles certain variants of breast cancer. There is always more to accomplish, but Goodman is content. After two decades of navigating patents and approvals, he is happy to know that an accessible Axumin® is positively affecting patients' lives.
Research on the radiotracer continues: Goodman, Jeffrey Olson and Schuster continue to investigate its use in detecting glioma, Schuster and Ashesh Jani, MD, professor of radiation oncology, are investigating its use in recurrent prostate cancer to guide decisions about radiation treatment; Baowei Fei, PhD, EngD, of the Coulter Department of Biomedical Engineering at Georgia Tech and Emory, is researching how to combine FACBC/Axumin® with ultrasound to guide prostate biopsy; and Emory urologist Martin Sanda, MD, and colleagues are investigating its use to assess cancer severity in high-risk prostate cancer.
Later support for clinical research on FACBC/fluciclovine came from the National Cancer Institute, the Georgia Research Alliance and the Georgia Cancer Coalition.
There is a series of blog pieces that accompany the patient centered video project linked above on this technology.
Press Release: FDA approves Emory-developed prostate cancer imaging probe
Urology: Axumin is the First FDA Approved Fluorinated PET Radiotracer for Prostate Cancer Staging