Timeline: Emory University's
Office of Technology Transfer
Celebrating 40 years of advancing Emory innovation from the lab bench to the marketplace.

For four decades, Emory’s Office of Technology Transfer (OTT) has bridged academic research and industry partnerships, ensuring that innovations leave the lab and maximize public benefit.
With more than 65 lifesaving products to reach the market and over $1.4 billion in cumulative revenue earned to sustain Emory's research and education enterprise, Emory OTT has grown into a leader in technology transfer, setting the standard for how universities can transform academic innovation into tangible solutions.
Scroll down to see the milestones that have defined our journey.
1985-1995
Laying the Foundation
After the passage of the Bayh-Dole Act in 1980, allowing universities to take ownership of their inventions made with federally funded research, the first technology transfer activity at Emory happened in 1981 with inventor David Lambeth’s first invention disclosure for Novel Compounds for Inhibition of Steroid Biosynthesis.
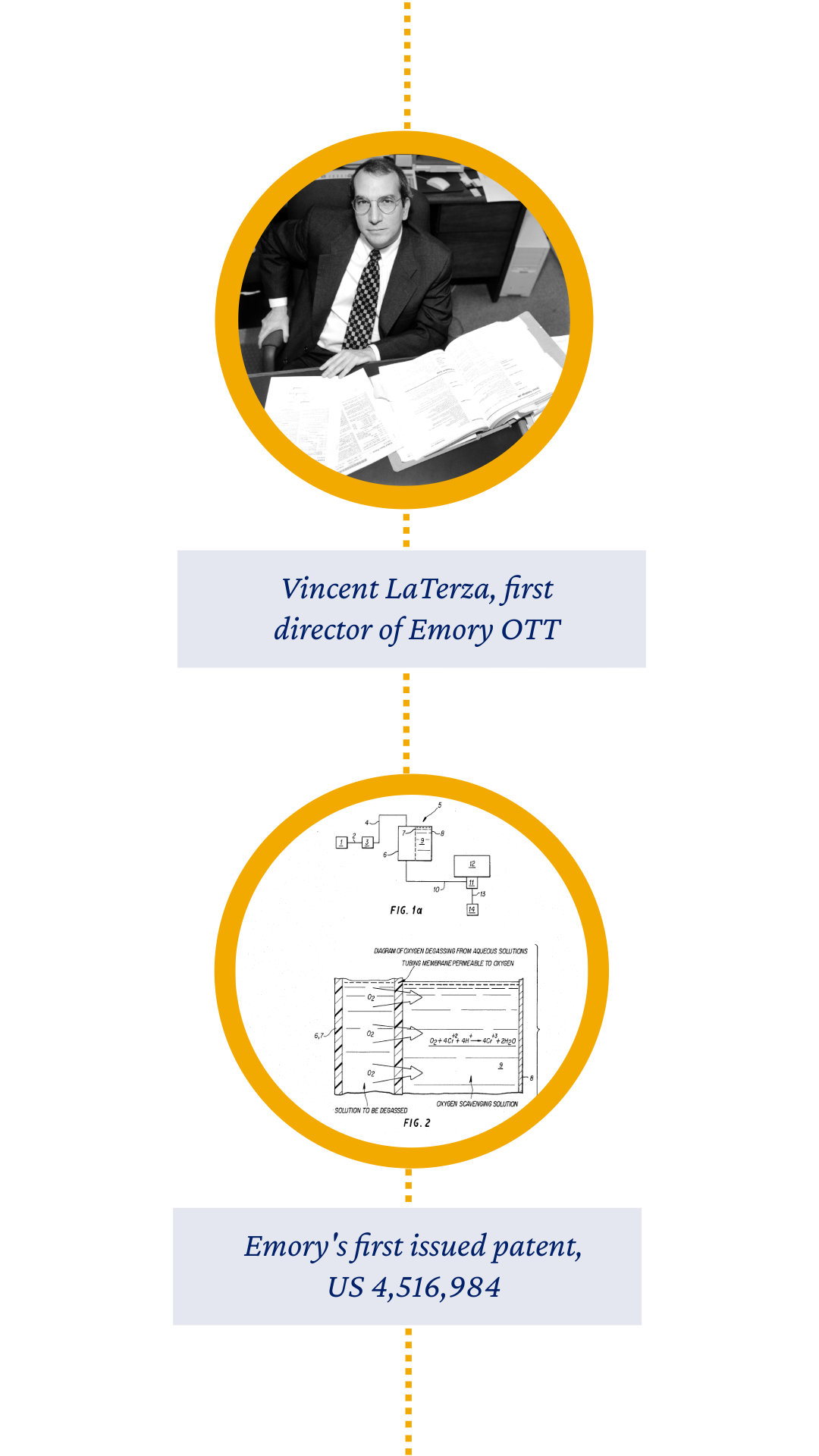
By 1985, Emory had earned its first U.S. patent — granted to Emory inventor Isiah Warner for a degassing process and apparatus for removal of oxygen. OTT also saw the formation of its first start-up company, CytRx (now LadRx), in 1986.
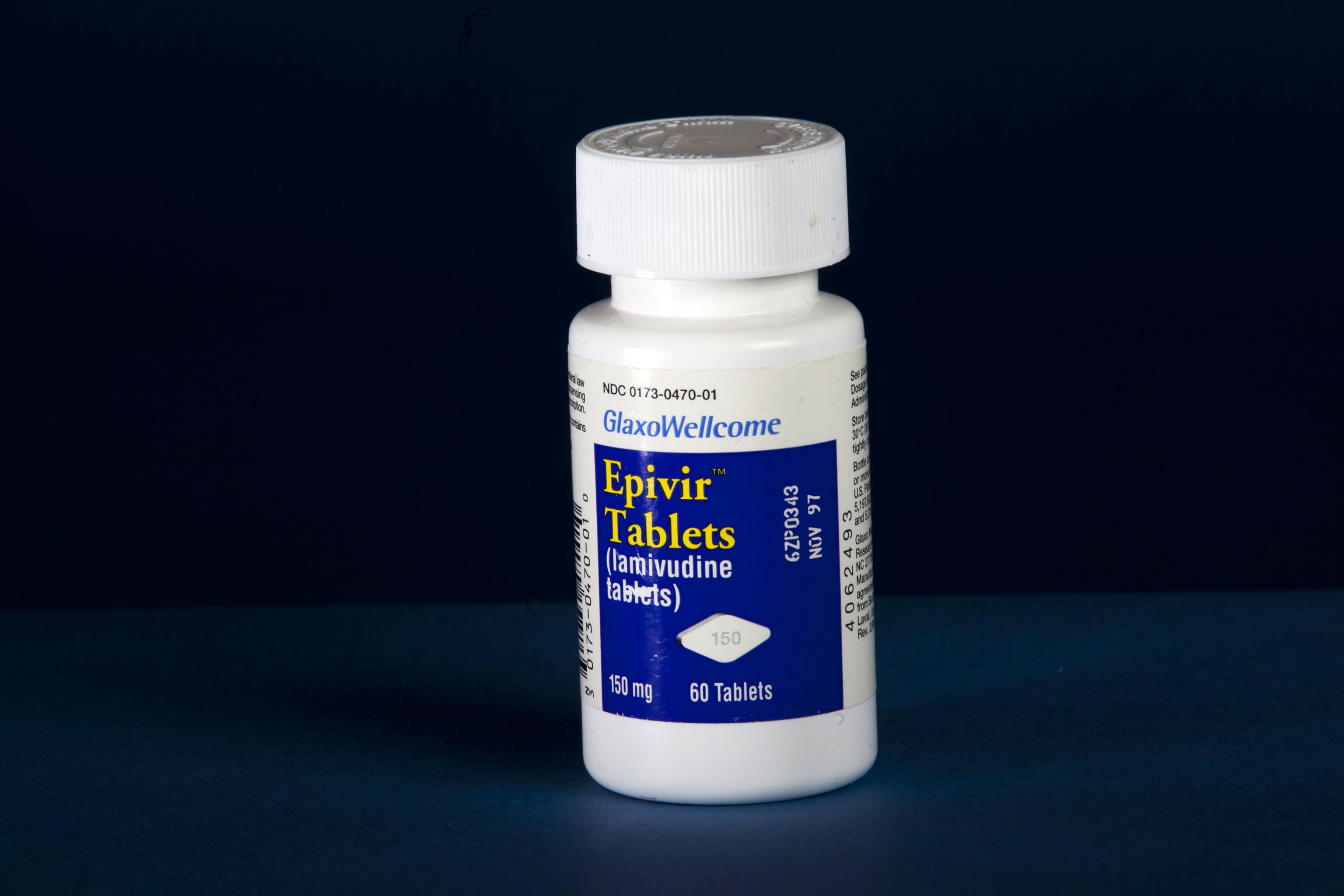
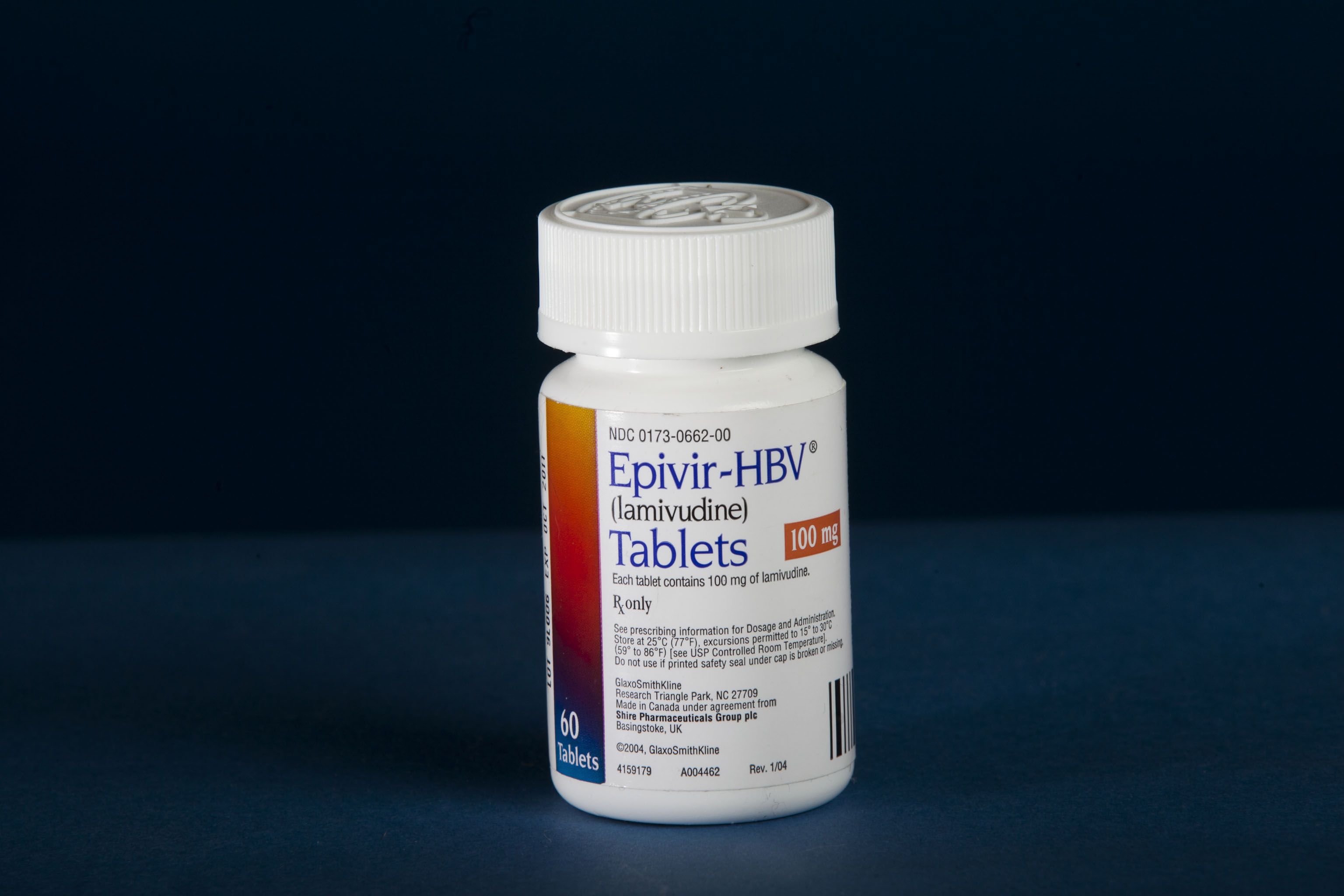
The '90s brought new momentum: Vincent LaTerza joined as the first dedicated OTT staff member in 1992. QuantEM became Emory’s first major product on the market in 1993. And in 1995, the FDA approved Epivir, an HIV treatment created by Emory researchers that would save the lives of millions worldwide.
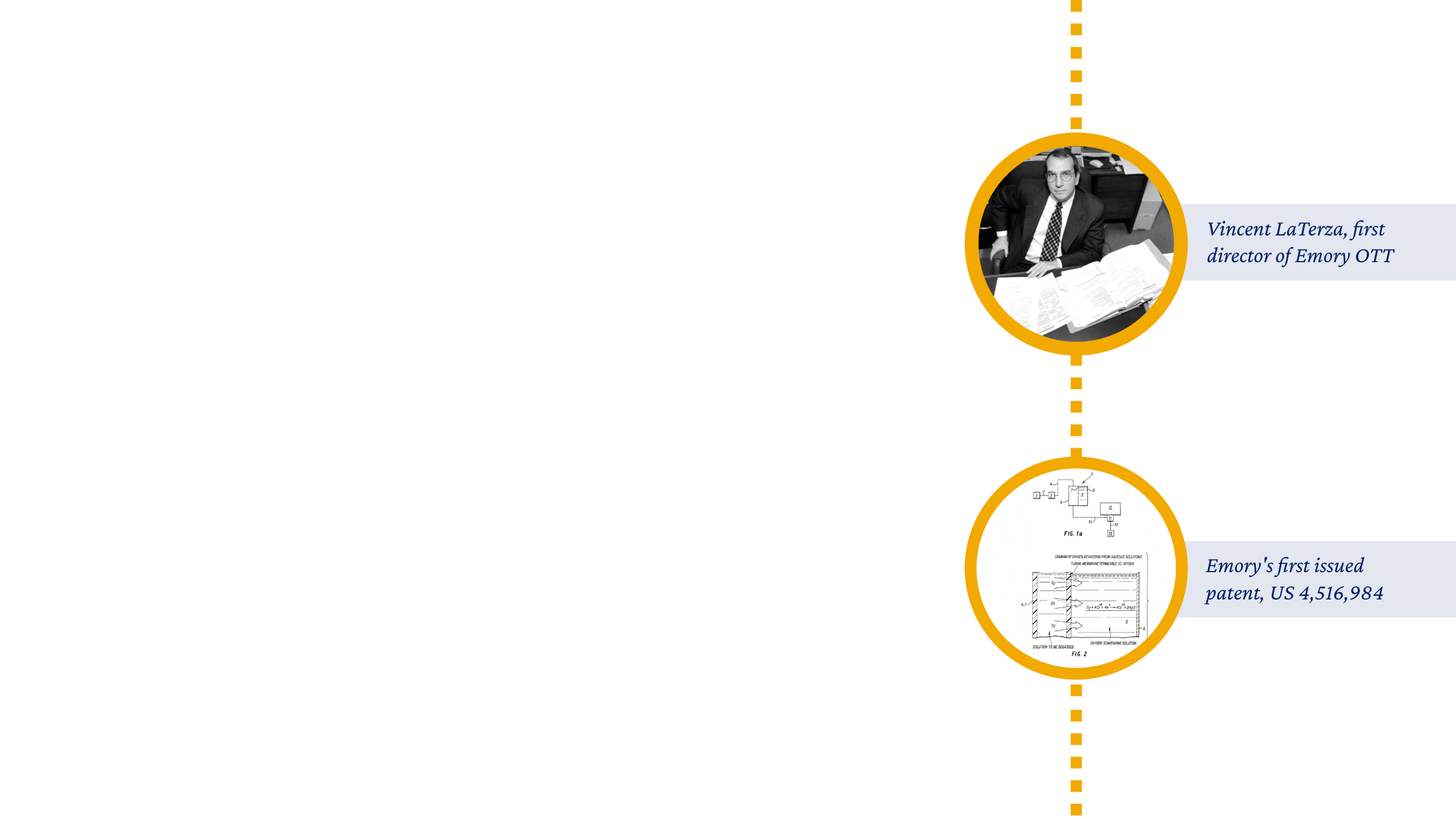
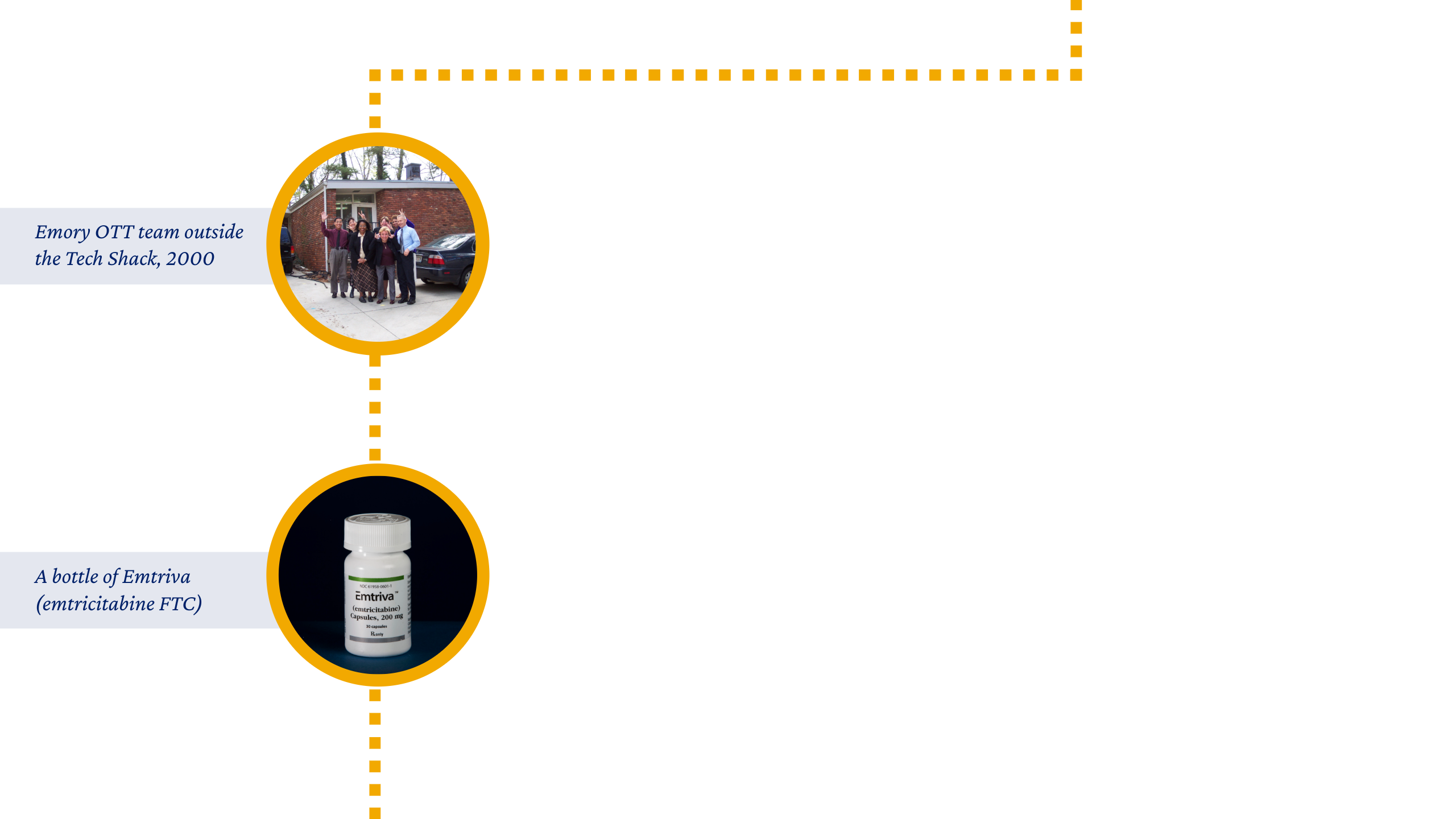
“When I started in 1995, there were only three of us in the office, located in a little house known as the ‘Tech Shack’ on Ridgewood Drive. It was run like a small company carved out of the university, with the expectation that we bring in dollars every year to fund research.”
Connie Newsome, OTT Office and Project Manager from 1995-2018
1995-2005
Growth and Independence
In 1997, the Office of Technology Transfer became an independent unit after spinning out of the Office of Sponsored Programs, reflecting Emory’s growing commitment to research translation.
Our second decade also brought more FDA approvals, the launch of OTT’s product pipeline, our 25th start-up, and our 1,000th invention disclosure. We also restructured our office to better meet Emory faculty needs, which included the implementation of a pre- and post-licensing value creation strategy, a position focused on processing MTAs, and a dedicated docketing position.
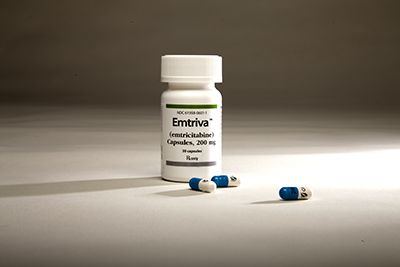
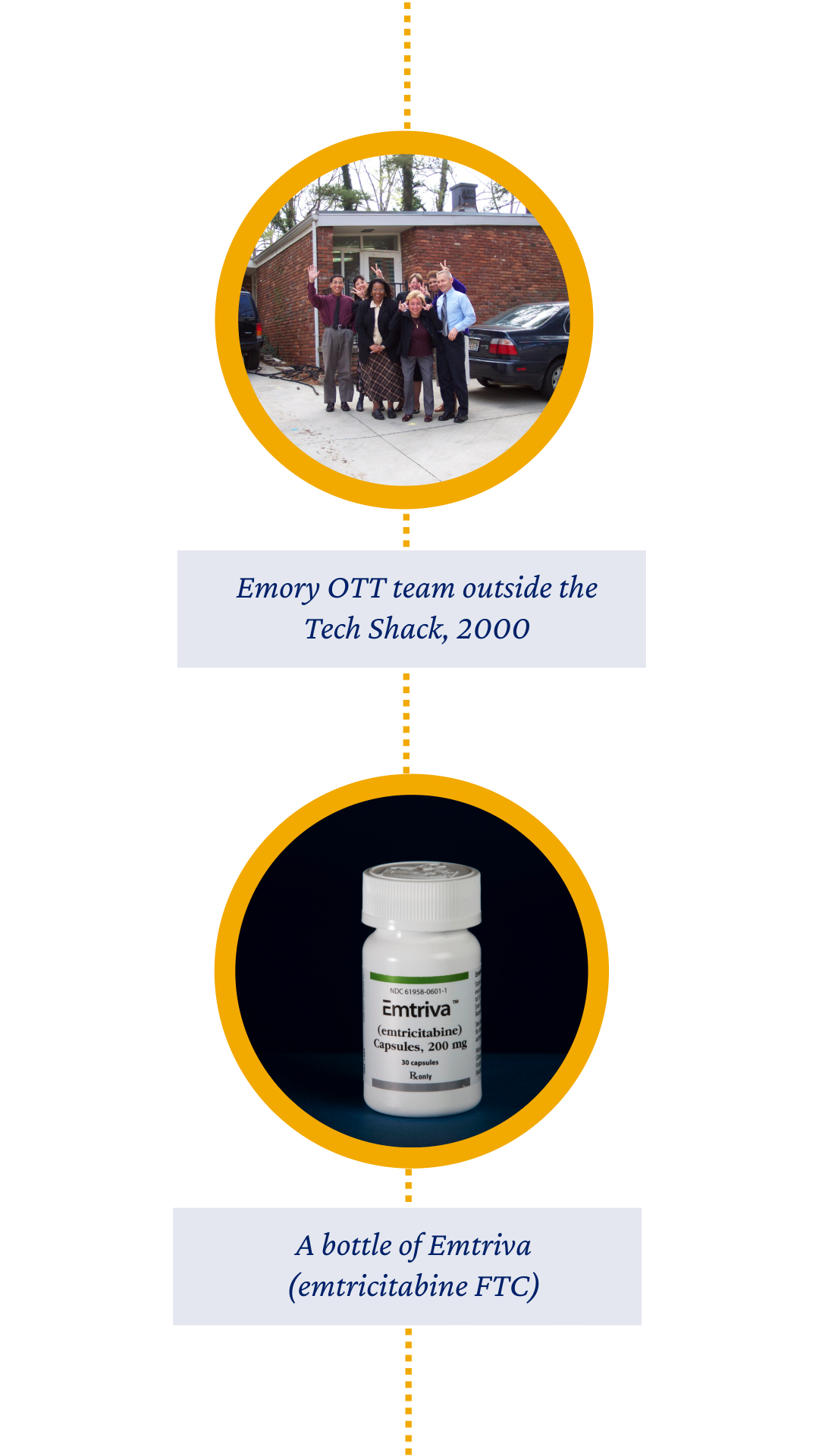
But the most notable event in our second decade came in 2005: Emory sold its future royalties for emtricitabine (FTC) for $540 million — the largest monetization in U.S. university history at the time — securing Emory’s place as a national leader in innovation.
Selected FDA Product Approvals for Emory Technologies — 1995-2005
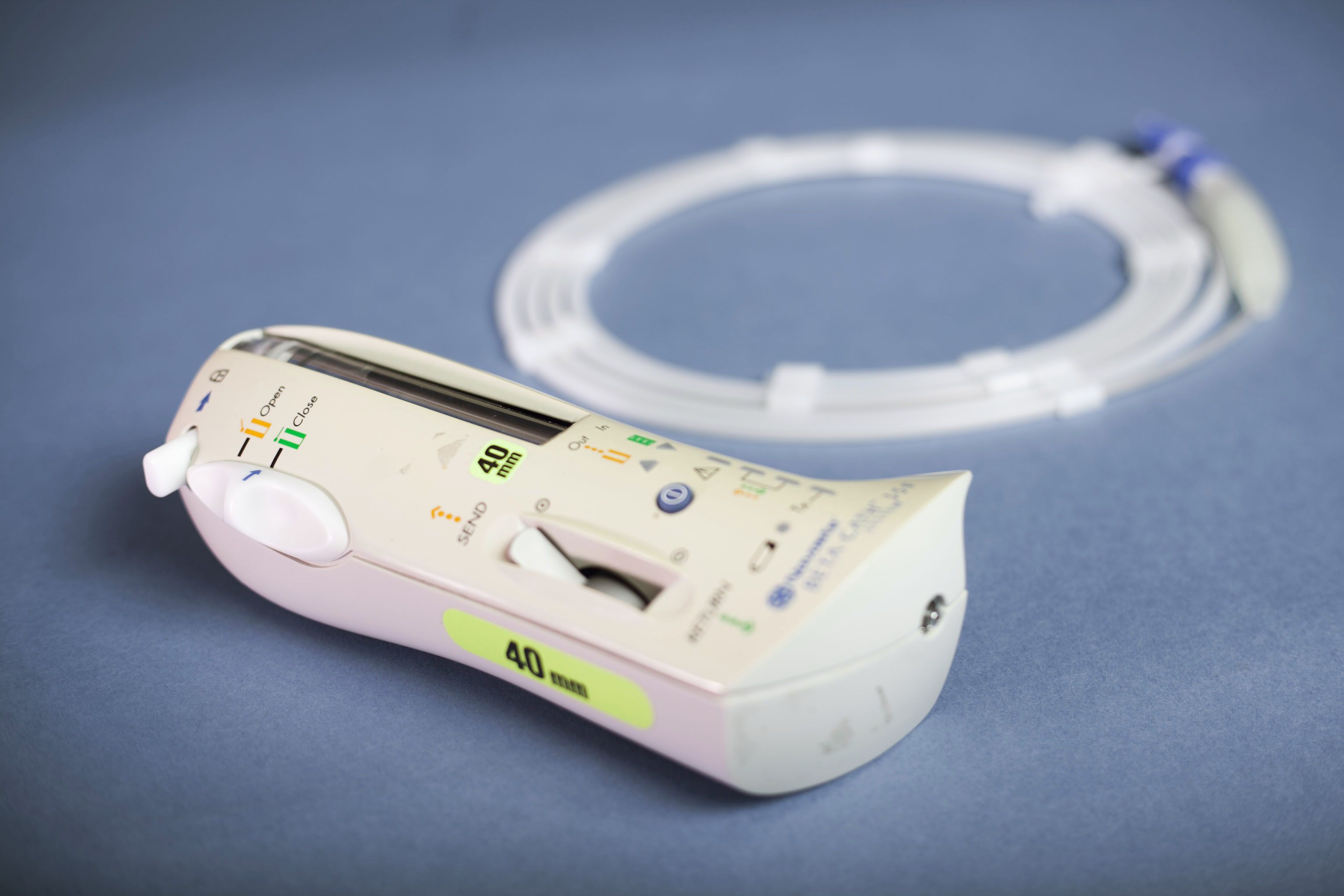
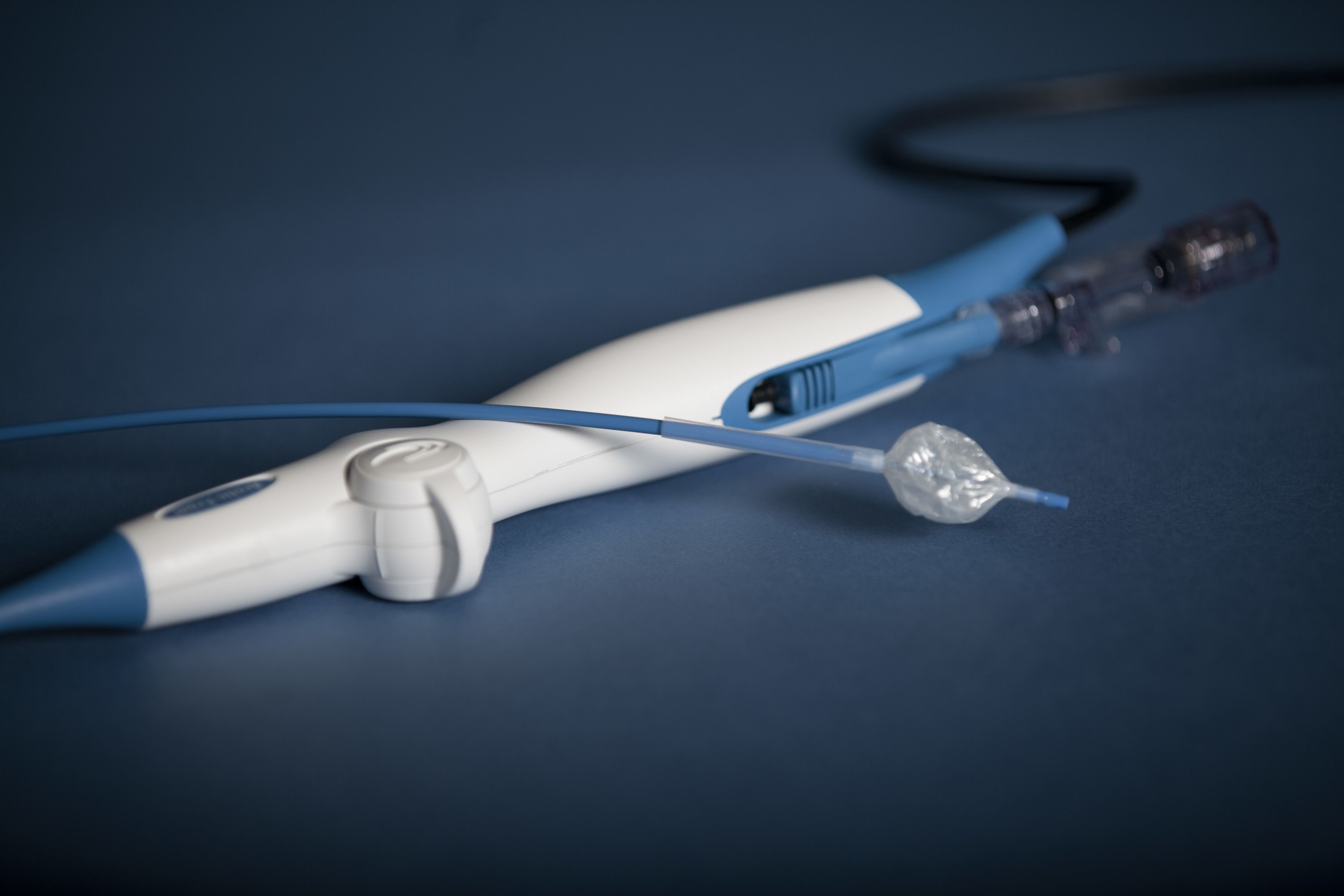
The Beta-Cath™ System, which uses vascular brachytherapy to prevent the artery from re-closing. Inventors: Ian Crocker, Spencer King, Keith Robinson, Ron Waksman.
The Beta-Cath™ System, which uses vascular brachytherapy to prevent the artery from re-closing. Inventors: Ian Crocker, Spencer King, Keith Robinson, Ron Waksman.
2005-2015
Expanding Impact
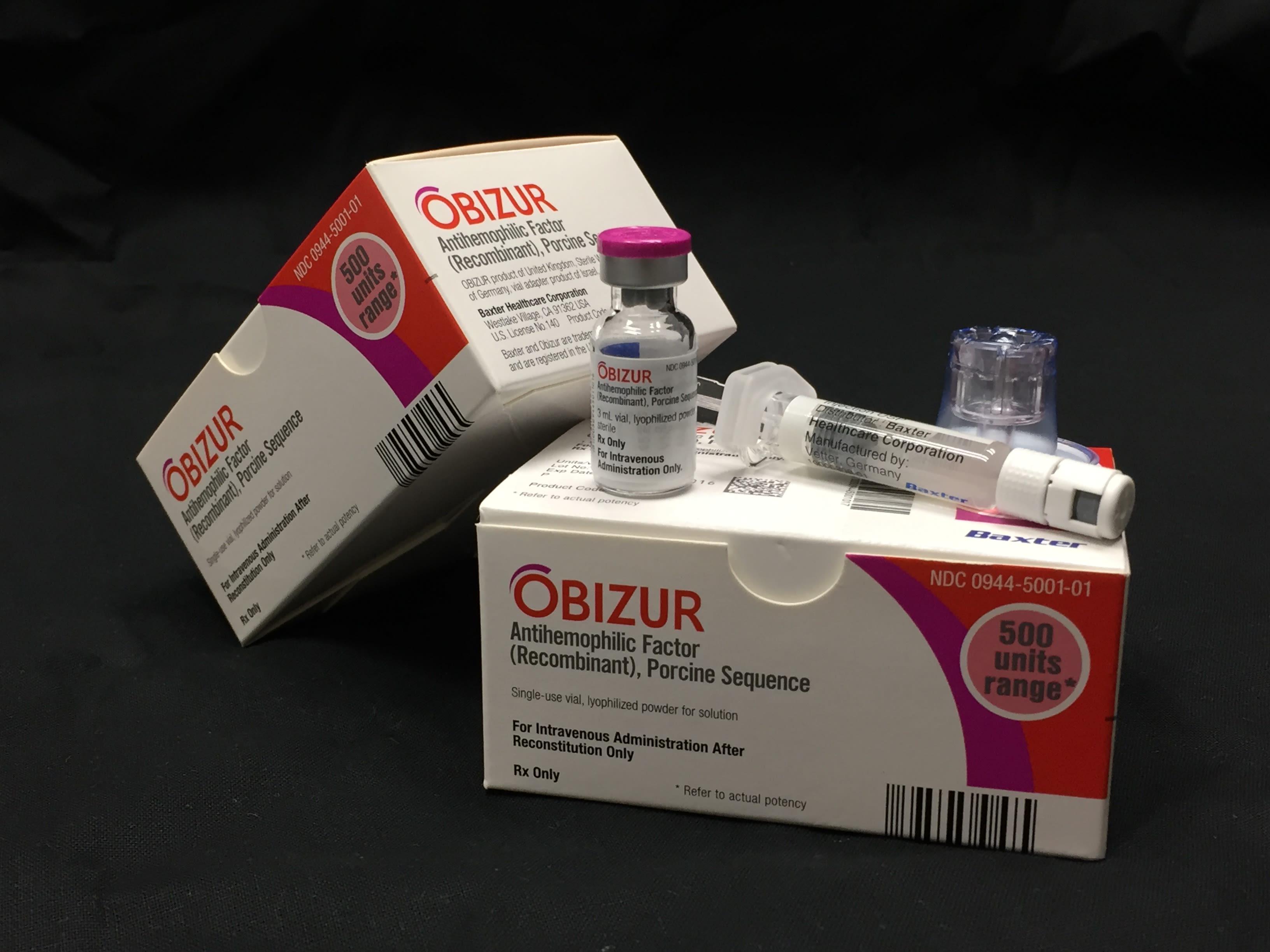
With a strong foundation in place, Emory entered a decade of rapid growth and global recognition. The university’s discoveries fueled a wave of FDA-approved products, including Velocity AI, a predictive imaging software for cancer (2007); NeuroStar TMS Therapy, an effective method of targeting treatment-resistant depression (2008); and Obizur, a treatment for hemophilia A (2015).
Emory OTT also had wins on campus. In 2006, we launched our licensing internship program, exposing graduate students to a rewarding career outside the lab. Our marketing and patent internships soon followed, launching in 2008 and 2010. Since then, we've prepared hundreds of interns for careers in technology licensing, IP law, and science communication.
In 2007, we hosted our first Annual Celebration of Technology and Innovation, honoring the researchers behind transformative ideas. While the first event featured a panel of innovators speaking on translational research, it's since evolved into an awards ceremony and pitch presentation.
We created the Emory Patent Group (EPG), our in-house team dedicated to filing and prosecuting patents, in 2010. EPG's creation has brought many benefits, including nearly $500,000 in annual savings and doubled patent filing outputs.
Finally, by the early 2010s, Emory ranked among the top 25 universities in licensing income according to AUTM, a reflection of our expanding impact on health, science, and society.


Selected FDA Product Approvals for Emory Technologies — 2005-2015
Goldshield® products, alcohol-free antibacterial solutions designed to protect against germs, available in sprays, foams, and wipes. Inventors: Gary Allred, Lanny Liebeskind.
Goldshield® products, alcohol-free antibacterial solutions designed to protect against germs, available in sprays, foams, and wipes. Inventors: Gary Allred, Lanny Liebeskind.
"We turn invention disclosures into patent applications."
Jim Mason, MS, JD, Patent Counsel in the Emory Patent Group
2015-2025
Global Leadership
Emory OTT continued to grow, launching new digital platforms — like techFeed and IdeaGate — to make discovery and collaboration more accessible than ever.
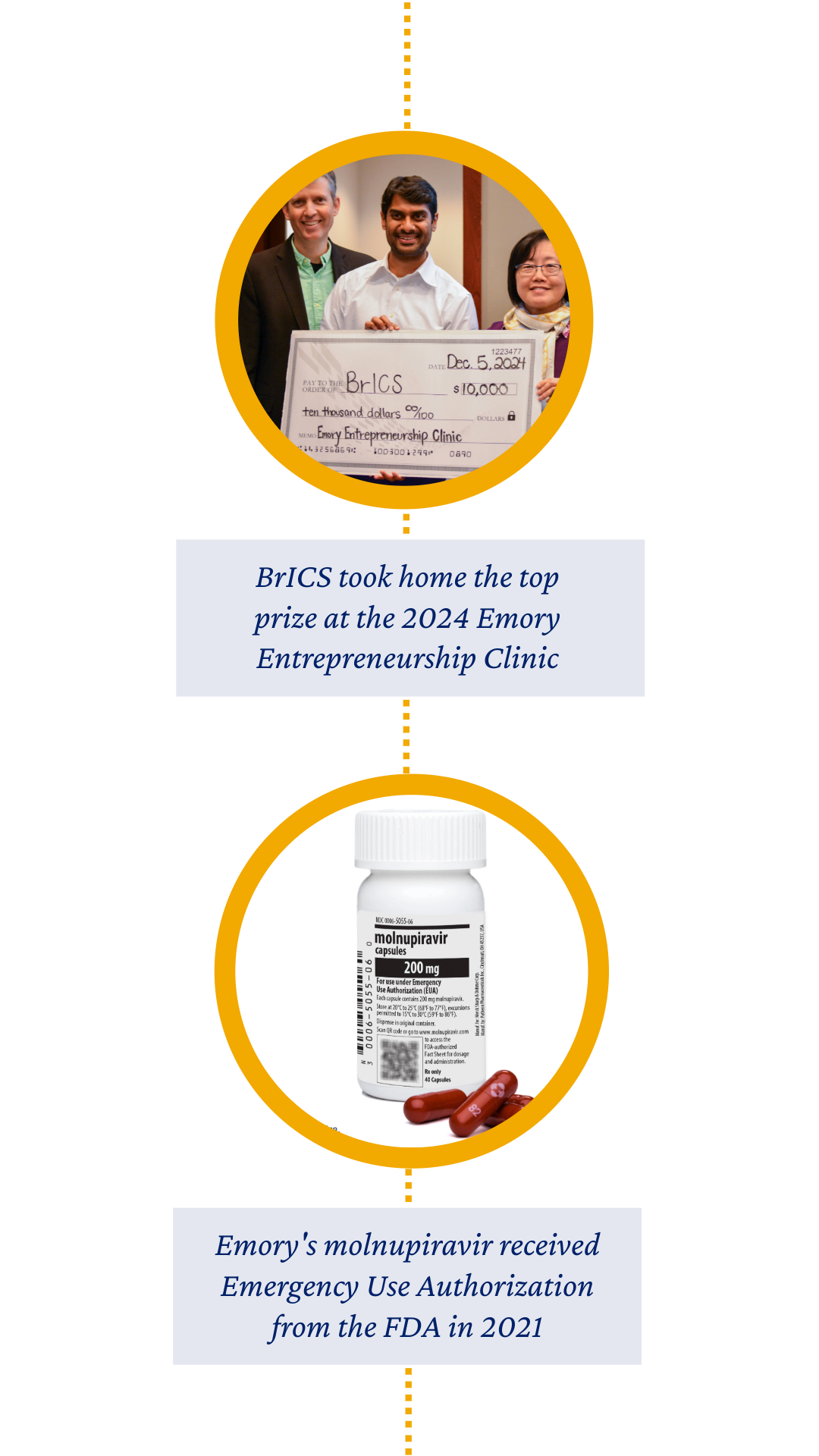
Breakthroughs also continued to happen, from the FDA approval of Axumin® in 2016 and XIPERE™ in 2021, to the 2021 rollout of LAGEVRIO™ (molnupiravir), a COVID-19 antiviral that reached patients across more than 100 countries.
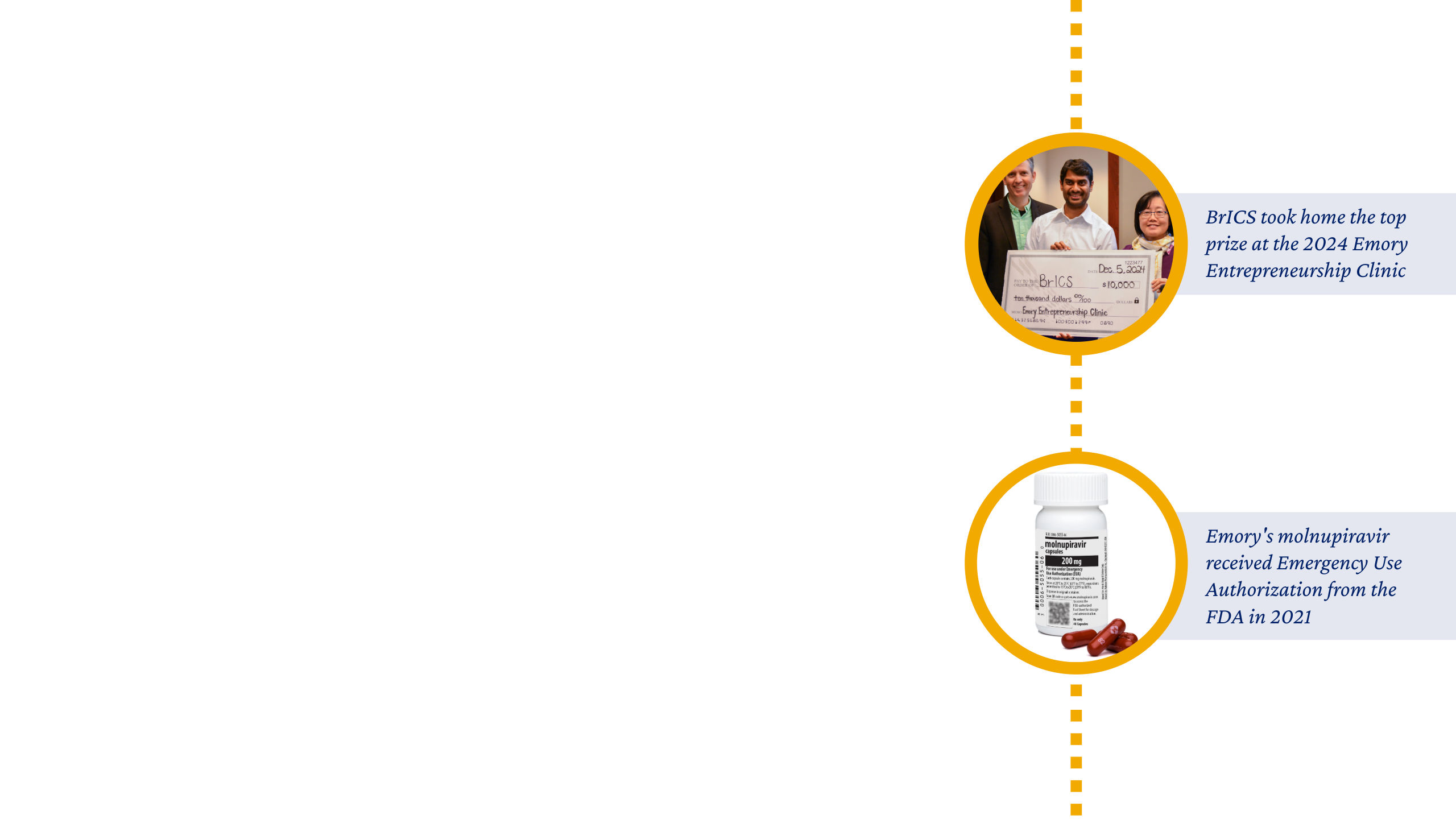
We also launched new faculty education programs, like the Emory Entrepreneurship Clinic and Faculty Founders Forum, to promote the spirit of entrepreneurship and innovation across campus.
Our fourth decade closed with national recognition and milestones: In 2023, Emory ranked as the third-top contributor of new drug discoveries by public institutions, in a study published in the Journal of Technology Transfer. This was also the first year we achieved $1 billion in sponsored research awards, and we continued to maintain top rankings in licensing revenue among American universities.
Selected FDA Product Approvals for Emory Technologies — 2015-2025
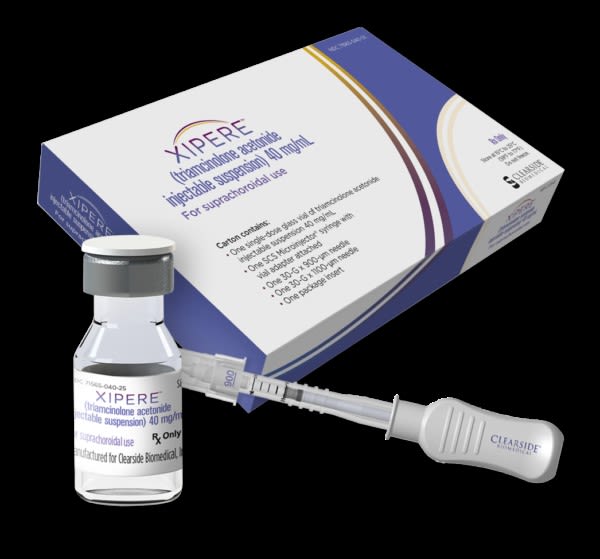
XIPERE™, a corticosteroid used to treat macular edema associated with an eye disease called uveitis. Inventor: Henry Edelhauser. (Photo courtesy of Clearside Biomedical, Inc.)
XIPERE™, a corticosteroid used to treat macular edema associated with an eye disease called uveitis. Inventor: Henry Edelhauser. (Photo courtesy of Clearside Biomedical, Inc.)
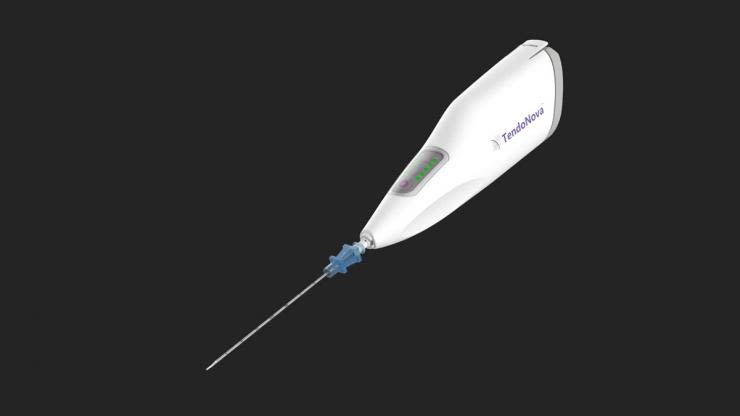
TendoNova® Ocelot, a device used in a micro-invasive procedure for treating chronic tendon pain. Inventor: Kenneth Mautner. (Photo courtesy of TendoNova.)
TendoNova® Ocelot, a device used in a micro-invasive procedure for treating chronic tendon pain. Inventor: Kenneth Mautner. (Photo courtesy of TendoNova.)
"With our stellar team, we’ve added value for faculty researchers and for the university through reducing technologies’ risk and increasing their potential viability."
Todd Sherer, Associate Vice President for Research and Executive Director of the Office of Technology Transfer
As we celebrate 40 years, we’re not just looking back — we’re looking forward. With new partnerships, entrepreneurial programs, and groundbreaking research, Emory continues to shape the future of innovation.
Most of the team at the Office of Research Administration's Research Week, October 2023.
Most of the team at the Office of Research Administration's Research Week, October 2023.