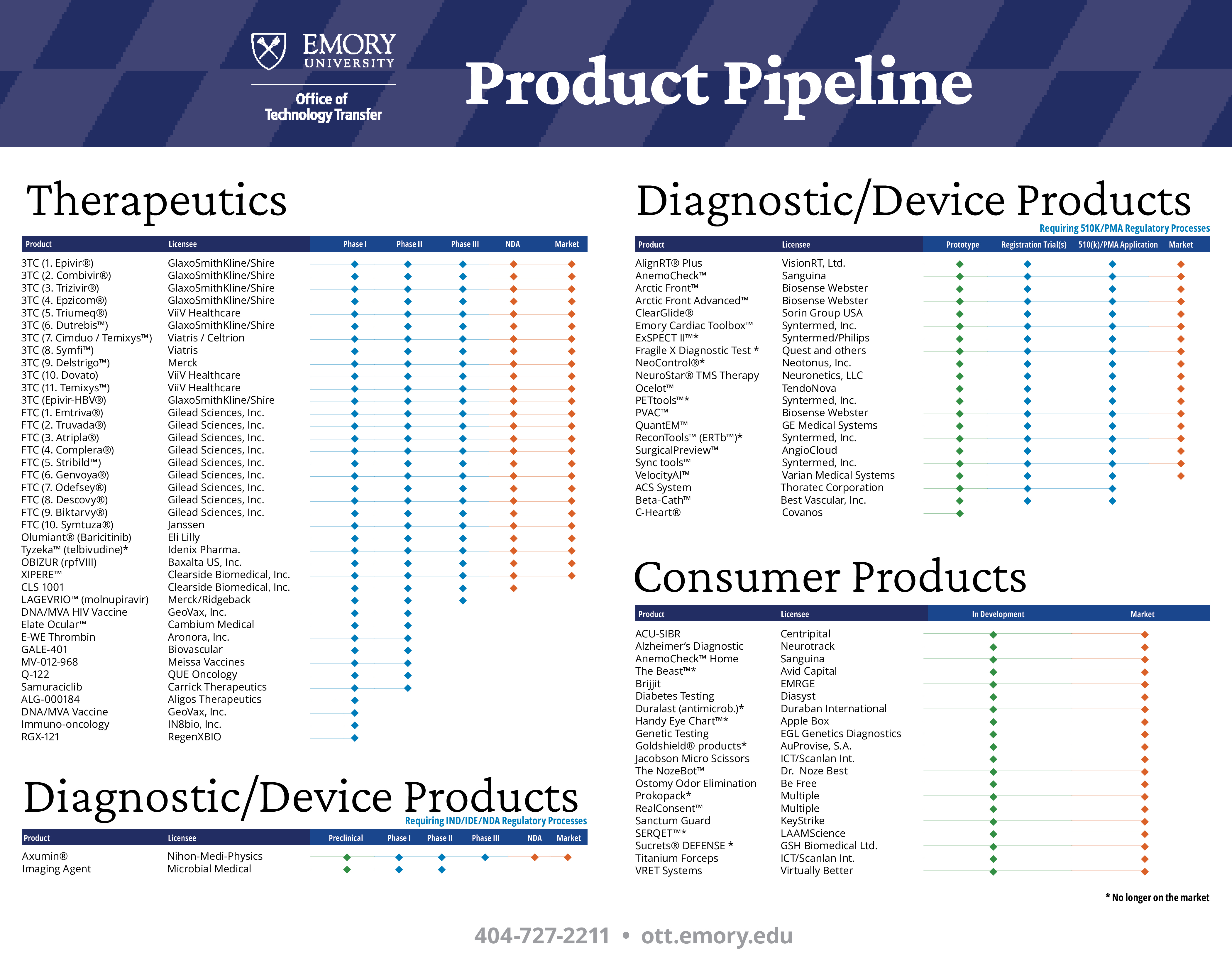
Product Pipeline
Success of a program centers on the number of products that have reached the market with our assistance. The ultimate validation for any technology transfer office is products on the market. Everybody likes to talk about patents filed and licenses negotiated, but that really isn't the end justification for technology transfer. It's completing the cycle of investing in research and having that effort improve people's lives. Emory has been fortunate to have over 65 products reach the market, with several having a significant, positive impact on society. Below see our list of products that have reached the market and promising discovery that we hope will.
| Product | Licensee | Indication | Preclinical | Phase I | Phase II | Phase III | NDA/BLA | Market |
|---|---|---|---|---|---|---|---|---|
| 3TC (1. Epivir®) | GlaxoSmithKline/Shire | HIV |  | |||||
| 3TC (2. Combivir®) | GlaxoSmithKline/Shire | HIV |  | |||||
| 3TC (3. Trizivir®) | GlaxoSmithKline/Shire | HIV |  | |||||
| 3TC (4. Epzicom®) | GlaxoSmithKline/Shire | HIV |  | |||||
| 3TC (5. Triumeq®) | ViiV Healthcare | HIV |  | |||||
| 3TC (6. Dutrebis™) | GlaxoSmithKline/Shire | HIV |  | |||||
| 3TC (7. Cimduo / Temixys™) | Viatris / Celtrion | HIV |  | |||||
| 3TC (8. Symfi™) | Viatris | HIV |  | |||||
| 3TC (9. Delstrigo™) | Merck | HIV |  | |||||
| 3TC (10. Dovato) | ViiV Healthcare | HIV |  | |||||
| 3TC (Epivir-HBV®) | GlaxoSmithKline/Shire | HBV |  | |||||
| FTC (1. Emtriva®) | Gilead Sciences, Inc. | HIV |  | |||||
| FTC (2. Truvada®) | Gilead Sciences, Inc. | HIV |  | |||||
| FTC (3. Atripla®) | Gilead Sciences, Inc. | HIV |  | |||||
| FTC (4. Complera®) | Gilead Sciences, Inc. | HIV |  | |||||
| FTC (5. Stribild™) | Gilead Sciences, Inc. | HIV |  | |||||
| FTC (6. Genvoya®) | Gilead Sciences, Inc. | HIV |  | |||||
| FTC (7. Odefsey®) | Gilead Sciences, Inc. | HIV |  | |||||
| FTC (8. Descovy®) | Gilead Sciences, Inc. | HIV |  | |||||
| FTC (9. Biktarvy®) | Gilead Sciences, Inc. | HIV |  | |||||
| FTC (10. Symtuza®) | Janssen | HIV |  | |||||
| Olumiant® (Baricitinib) | Eli Lilly | COVID-19 |  | |||||
| Tyzeka™ (telbivudine)* | Idenix Pharma. | HBV |  | |||||
| OBIZUR (rpfVIII) | Baxalta US, Inc. | Hemophilia |  | |||||
| XIPERE™ | Clearside Biomedical, Inc. | Macular Edema |  | |||||
| CLS 1001 | Clearside Biomedical, Inc. | Drug Delivery |  | |||||
| LAGEVRIO™ (molnupiravir) | Merck/Ridgeback | COVID-19 |  | |||||
| DNA/MVA HIV Vaccine | GeoVax, Inc. | HIV/Prophylactic |  | |||||
| Elate Ocular™ | Cambium Medical | Dry Eye |  | |||||
| E-WE Thrombin | Aronora, Inc | Ischemic Stroke/ Heart Attack |  | |||||
| GALE-401 | Biovascular | Thrombocythemia |  | |||||
| MV-012-968 | Meissa Vaccines | Respiratory Syncytial Virus |  | |||||
| Q-122 | QUE Oncology | Hot Flashes |  | |||||
| Samuraciclib | Carrick Therapeutics | Breast Cancer |  | |||||
| ALG-000184 | Aligos Therapeutics | HBV |  | |||||
| DNA/MVA Vaccine | GeoVax, Inc. | HIV/ Therapeutic |  | |||||
| Immuno-oncology | IN8bio, Inc. | Cancer |  | |||||
| RGX-121 | RegenXBIO | Hunter Syndrome |  | |||||
| Anti-CD40 Antibody | Kiniksa Pharmaceuticals | Transplant Rejection/ Autoimmune |  | |||||
| EIDD-1931 | DRIVE | Emerging Disease |  | |||||
| Hemodialysis System | Baxter Bioscience | Kidney Failure/ Dialysis |  | |||||
| NMDAR-R2B Blocker | NeurOp Corporation | Ischemia/ Pain |  | |||||
| r13 (7,8-DHF prodrug) | Shanghai Braegen | Alzheimer’s/ Pain |  | |||||
| Product | Licensee | Indication | Prototype | Registration Trial(s) | 510(k)/PMA Application | Market | ||
|---|---|---|---|---|---|---|---|---|
| AlignRT® Plus | VisionRT, Ltd. | Radiation Therapy |  | |||||
| AnemoCheck™ | Sanguina | Anemia |  | |||||
| Arctic Front™ | Biosense Webster | Atrial Fibrillation |  | |||||
| Arctic Front Advanced™ | Biosense Webster | Atrial Fibrillation |  | |||||
| Beta-Cath™ | Best Vascular, Inc. | Restenosis |  | |||||
| ClearGlide® | Sorin Group USA | Vein Harvesting |  | |||||
| Emory Cardiac Toolbox™ | Syntermed, Inc. | Cardiac Imaging |  | |||||
| ExSPECT II™* | Syntermed/Philips | Cardiac Imaging |  | |||||
| Fragile X Diagnostic Test * | Quest and others | Fragile X Syndrome |  | |||||
| NeoControl®* | Neotonus, Inc. | Incontinence |  | |||||
| NeuroStar® TMS Therapy | Neuronetics, LLC | Depression |  | |||||
| Ocelot™ | TendoNova | Tendinopathy |  | |||||
| PETtools™* | Syntermed, Inc. | Cardiac Imaging |  | |||||
| PVAC™ | Biosense Webster | Atrial Fibrillation |  | |||||
| QuantEM™ | GE Medical Systems | Renal Imaging |  | |||||
| ReconTools™* (ERTb™) | Syntermed, Inc. | Cardiac Imaging |  | |||||
| SurgicalPreview™ | AngioCloud | Vascular Imaging |  | |||||
| Sync tools™ | Syntermed, Inc. | Cardiac Imaging |  | |||||
| VelocityAI™ | Varian Medical Systems | Oncology Imaging |  | |||||
| ACS System | Thoratec Corporation | Vascular Surgery |  | |||||
| C-Heart® | Covanos | Cornary Software |  | |||||
| Product | Licensee | Indication | Preclinical | Phase I | Phase II | Phase III | NDA | Market |
|---|---|---|---|---|---|---|---|---|
| Axumin® | Nihon-Medi-Physics | Tumor Imaging |  | |||||
| Imaging Agent | Microbial Medical | Bacterial Infection |  | |||||
| Product | Licensee | Indication | In Development | Market | ||
|---|---|---|---|---|---|---|
| ACU-SIBR | Centripital | Hospital Rounds |  | |||
| Alzheimer’s Diagnostic | Neurotrack | Alzheimer’s Diagnostic |  | |||
| AnemoCheck™ Home | Sanguina | Alzheimer’s Diagnostic |  | |||
| The Beast™* | Avid Capital | OR Table Attachment |  | |||
| Odor Elimination System | Be Free Technologies | Ostomy |  | |||
| Brijjit | EMRGE | Wound Closure & Healing |  | |||
| Diabetes Testing | Diasyst | Diabetes |  | |||
| Duralast (antimicrob.)* | Duraban International | Antimicrobial Coating |  | |||
| Genetic Testing | EGL Genetics Diagnostics | Genetic Testing |  | |||
| Goldshield® products* | AuProvise, S.A. | Antimicrobial Shield |  | |||
| Handy Eye Chart™* | Apple Box | Vision Testing |  | |||
| Jacobson Micro Scissors | ICT/Scanlan Int. | Vascular Surgery |  | |||
| The NozeBot™ | Dr Noze Best | Nasal Congestion |  | |||
| Prokopack* | Multiple | Bug Catcher |  | |||
| RealConsent™ | Multiple | Sexual Assault Training |  | |||
| Sanctum Guard | Keystrike | Cybersecurity |  | |||
| SERQET™* | LAAMScience | Antimicrobial Coating |  | |||
| Sucrets® DEFENSE * | GSH Biomedical Ltd. | Immune System Boost |  | |||
| Titanium Forceps | ICT/Scanlan Int. | Surgery |  | |||
| VRET Solutions | Virtually Better | Virtual Reality Therapy |  | |||
* No longer on the market
Note: A non-exhaustive list of technologies/products that have not received market approval.
© Emory University, Office of Technology Transfer