Gene Mutations & Dementia
Understanding How Gene Mutations Cause Dementia: Developing Therapeutics for Age-Associated Neurodegenerative Diseases
Thomas Kukar, PhD, associate professor in the Department of Pharmacology and Chemical Biology in the School of Medicine at Emory, has had a long-standing—and personal—interest in the causes of age-associated neurodegenerative diseases. "My grandfather had Alzheimer's disease," he says. "It was terrible to watch growing up, but I became fascinated with trying to understand the underlying mechanisms."

Thomas Kukar
In 2010, his research at Emory began to focus on how mutations in the progranulin gene (GRN) increase the risk of Alzheimer's or cause frontotemporal dementia, a fatal brain disease that is the most common cause of dementia in people under age 60. "I call frontotemporal dementia the most common dementia that no one's ever heard of," says Kukar.
The GRN gene provides instructions for making a protein called progranulin that is involved in cell growth, wound repair, and inflammation. Progranulin is found in tissues throughout the body as well as several types of brain cells, including nerve cells (neurons) and microglia (the immune cells of the brain). Progranulin is especially important for the health and survival of neurons.
Mutations in the GRN gene, which provides instructions to make progranulin, were discovered to cause frontotemporal dementia in 2006. "But no one knew exactly what progranulin did in the brain or cells," Kukar says. "GRN mutations prevent the body from making sufficient amounts of progranulin, which ultimately causes neurons to die."
Kukar's hope was to better understand the normal function of progranulin, to develop drugs that correct what is missing. "This mystery seems to hinge on a simple question," he says, "but it's been a long and exciting journey."
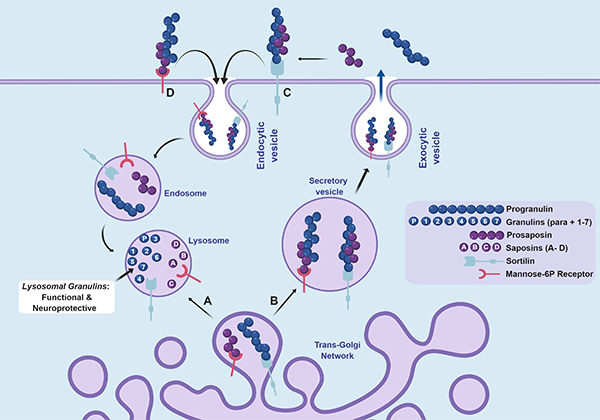
Figure 1. Overview of progranulin (PGRN) trafficking and production of neuroprotective granulins (GRNs) in the lysosome. (More details in the note below.)
Studying the genes of patients who died from frontotemporal dementia has been useful. "Because frontotemporal dementia is often genetic, autosomal dominant and inherited in families, you can track and identify common genetic causes," he says. "In the vast majority of Alzheimer's cases, there's not a single genetic cause."
Indeed, by sequencing the genome of many families with a history of frontotemporal dementia, researchers have discovered more than 100 mutations in the progranulin gene that cause frontotemporal dementia, and some that increase the risk of developing Alzheimer's. All of the mutations decrease the levels of progranulin that circulate in the body.
"The big question has been, what does progranulin do in the brain and why does the loss of it lead to neurodegeneration and dementia?" Kukar says. Progranulin is a large protein composed of seven different subunits called granulins. Granulins are made from the breakdown of full-length progranulin, but it was unclear where this happens. Additionally, some reports suggested that granulins themselves are toxic and cause inflammation.
"Our major discovery is that granulins are made in the lysosome," Kukar says. "That's important because we think that's where granulins function. Additionally, we find granulins are actually beneficial for cells and not toxic. Our data suggest that the decrease in granulin function in the lysosome leads to disease."
Lysosomes, he says, are the "recycling center" of the cell and are important in maintaining the cell's overall health and fitness. When lysosomes are impaired, that can lead to many devastating diseases. The brain is particularly affected because neurons have to live for a very long time, and they rely on the lysosome pathway to keep things functioning properly. "We think it starts to break down with age and time," Kukar says, "and the lack of granulins leads to a decrease in the activity of lysosomes, causing things like fats and sugars to accumulate, which cause inflammation and make the brain sick."
The mechanism of neurodegenerative diseases caused by GRN mutations involves a balance between progranulin and production of these granulin segments. It follows, then, that granulins could be used as a therapeutic. "We have narrowed it down to two or three segments that are especially useful," he says. "We can potentially develop optimized versions of these proteins that traffic to the brain, and administer them like insulin."
The goal is to use granulin proteins as therapeutics for age-related neurodegenerative diseases such as Alzheimer's and frontotemporal dementia, both of which share lysosome dysfunction and inflammation. This idea is being tested in mouse models of frontotemporal dementia. Ideally, the results will translate into treating humans who have decreased levels of granulins and are at risk for developing dementia.
Note: Figure 1. Overview of progranulin (PGRN) trafficking and production of neuroprotective granulins (GRNs) in the lysosome. PGRN is a glycoprotein that traffics through the trans-golgi network and A) directly routed to the lysosome or B) secreted through exocytosis. PGRN can be endocytosed through the C) sortilin receptor and routed to lysosomes where PGRN is cleaved into GRN proteins (para and 1 through 7) by cathepsin proteases. PGRN also binds the prosaposin (PSAP) protein, which then binds the D) mannose-6-phosphate receptor to mediate lysosomal trafficking of both proteins. PSAP is processed into saposin (A-D) proteins that facilitate sphingolipid breakdown in the lysosome. We find that granulins are stable in the lysosome and suggest they have a beneficial and neuroprotective function. The precise function of granulins in the lysosome is unknown and is an active area of investigation in the Kukar lab.
Read our technology brief
Techid: 16127