Small Molecules & Hemangiomas
Small Molecules: Vascular Malformations and Stereoisomers
Treating Hemangiomas in Infants
The developed adult body, laid end to end, has almost 100,000 miles of blood vessels packed in it. They grow as you do, from fifteen days after fertilization through the developments of the body, providing blood to the whole of your body. Their growth is tightly controlled by a whole host of mechanisms and molecular switches, developed through generations of evolutionary history. But as your blood vessels replicate countless times, things have the potential to go wrong. Signaling pathways that usually allow for normal growth spin out of control, creating cells from existing blood vessels where none should be in uncontrolled angiogenesis.
At birth, these developmental problems generally become clear. One type are hemangiomas of infancy, which affect some 10% of children; they are generally harmless red birthmarks that disappear with time, but sometimes, depending on their placement, can interfere with vision or breathing, But other related vascular diseases, including vascular lesions and cancers, have the ability to grow throughout the body and kill patients as they get older. Clinicians have used beta-blockers, a class of drugs initially developed to prevent heart attacks and lower blood pressure, to treat these diseases, as they also have proven to effectively slow down or reverse this blood vessel overgrowth — but lowering blood pressure is a dangerous gamble when treating infants. How, then, to treat these problems?
That's where Jack Arbiser's lab comes in. By utilizing a selective formulation of propranolol, one of the first beta-blockers ever synthesized, his team has been able to mimic the properties of beta-blockers that stop blood vessel growth without the risky side-effects.
Jack Arbiser, MD, PhD, Professor of Dermatology, is an Emory BS/MS alum and Harvard Medical School graduate and has been developing small-molecule treatments for disease for most of his career. He has been "following the propranolol story from the beginning." About 10 years ago, research began to emerge on the potential anti-angiogenic properties of propanolol. Treatments in patients with heart conditions showed unexpected anti-angiogenic side effects, and large-scale statistical studies revealed people who take propranolol on average had less cancers. But it was Arbiser's team that helped articulate the chemical underpinnings of this effect.
To understand, a quick organic chemistry refresher: All molecules are made up of atoms, which are all connected to each other in a specific order in three-dimensional space. At certain single atoms (chiral centers), where all the atoms connected to it are different, you can change the structure of the molecule by switching where two groups are on the molecule. These two structures are called stereoisomersof each other. They differ in the spatial orientation, the 3-D placement,of their atoms. These two "stereoisomers" may be made up of the exact same elements, connected to the exact same other elements. But because everything is differently spatially arranged, they're different molecules – potentially with different properties, as well.
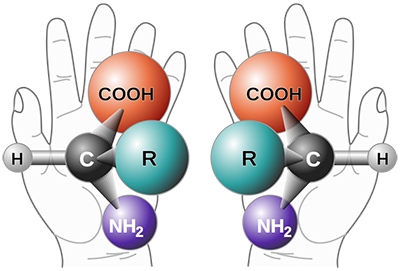
Two different “stereoisomers.” These molecules are made up of the exact same elements, connected to the exact same elements – but they’re not the same molecule because they’re differently arranged in space. Think about your left hand versus your right: they’re both made of the same parts, but you can’t put one over the other. Graphic courtesy of Wikipedia Commons.
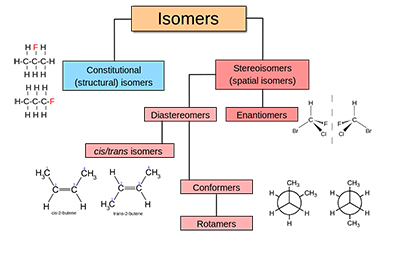
A chart explaining the different types of isomerism. Two molecules can be made up of the exact same elements but look very different. Graphic courtesy of Wikipedia Commons.
Just like a lot of biological and medical compounds, propranolol actually refers to a couple of "different" molecules – two stereoisomers. It's usually clinically administered in a liquid form as a mixture of these two stereoisomers. But, as it turns out, one of the spatial arrangements of propanolol, is more active against angiogenesis and is not a beta-blocker. By giving patients a dose of only this molecule, you can have the same anti-angiogenic treatment without the beta-blocker side effects. And since this molecule (in both forms) has already seen use around the world, it potentially could get through the FDA more quickly and onto the market much faster than competing treatments.
“Small solutions can make a big difference, as we can see in Dr. Arbiser’s technology. They’ve found a simple solution to a complex issue," said Mark Coburn, Director, Licensing at Emory OTT.
Arbiser's team is also testing other beta-blockers similar in structure to propranolol, each with stereoisomers that may end up having similar functions to (R)-propranolol. As for the future of treating vascular disorders on the whole? Arbiser is a firm believer in the power of small molecules. While many developmental therapeutics are relying on the power of very complex, "large" molecular structures that can very precisely target certain mechanisms of disease, it's usually very hard to synthesize a lot of that molecule. "If you want to use [a] molecule for thousands of people, you've got to make thousands of pounds of it. It's like if you want to make a fine watch in Geneva, Switzerland, but if you need to make a million of them … there's a reason why a Rolex costs more than a Timex." Finding drug targets that can be affected by molecules less than a nanometer in size (and then designing them) is no small task, but it's a strategy that's produced most medications we have today.
Techid: 18179Read our technology brief