Treating Cancer Patients with Comorbid Atherosclerosis
Slaying Two Monsters with One Sword

Lily Yang is the Nancy Panoz Chair of Surgery in Cancer Research, Department of Surgery at Emory University School of Medicine.
Stephen Nowland/Emory University
There’s a tradeoff in cancer management: Many current cancer immunotherapies can target tumors, but they can also induce the progression or development of atherosclerosis by inducing systemic immune-related side effects. Ultimately, there is a need for therapy that can effectively treat cancer and also help in the treatment of cardiovascular diseases. Fortunately, Emory professor of surgery and radiology Lily Yang, MD, PhD, and her team of researchers may have a solution.
The Problem with Current Cancer Therapeutics
Current targeted cancer treatments are delivered intravenously and therefore systemic – meaning the treatments spread throughout the body instead of targeting the cancerous area. And while the combination of cancer immunotherapy and chemotherapy enhances a tumor's sensitivity to the drugs, the medicine's use of blocking antibodies also weakens the patient’s own immunosuppressive cells. This can open the door to other problems, including new or worsened diseases.

Surgical Oncology Nanomedicine Research Lab members Kory Wells (left) and Songyu Wu, PhD.
Lily Yang
Yang says that many cancer patients are in an aging population and tend to have comorbid cardiovascular diseases or develop them during their treatment. This happens because the systemic treatment that courses through a patient’s entire body, while toxic to cancer cells, can also impact vital organs.
“We’re observing more and more side effects of cancer treatments that cause other problems or make the existing health problems even worse,” Yang says. “Some treatments induce cardiovascular problems. They might kill the patient before they kill the cancer.”
People who have cardiovascular disorders also have a higher risk of developing cancer.
“Lots of cancer patients already have cardiovascular problems, and when they are treated with immunotherapeutic agents, they will have some tumor response, but the cardiovascular problem will also become worse,” Yang explains.
Treating Cancer and Atherosclerosis with Drug-Filled Nanoparticles
To tackle the problem of concurrent diseases, Yang and her team are developing a new immunotherapy agent that has a stronger tumor response (i.e., will be more effective at eradicating the cancerous cells) while reducing the cardiovascular side effects.
The team uses a hyaluronic acid nanoparticle agent (HANP) as the delivery system for their therapeutic. This nanoparticle agent is composed of a material found within the human body, known as hyaluronic acid. Hyaluronic acid is a naturally occurring sugar molecule that can be used to treat burns and skin sores, moisturize eyes in the form of eye drops, and hydrate and plump the skin in cosmetic applications. From these uses, we know that hyaluronic acid has clinical applications and is proven to be a safe material for the human body in that it is biocompatible and biodegradable.
According to Yang, “We used that material as a drug carrier to immunotherapy agents, such as immune checkpoint blocking peptides. Inside that nanoparticle, we can encapsulate drugs to treat cancer and activate immune responses.”
The team’s drug of choice? A cholesterol-inhibiting drug already approved by the FDA for clinical trials.
Avasimibe: Reducing Cholesterol and Preventing Cancer Proliferation
Avasimibe is a drug that reduces cholesterol in the blood and plaque on the artery walls. Because cancer cells need cholesterol to proliferate, Avasimibe works on two levels by preventing tumor growth and plaque development that leads or contributes to atherosclerosis.
An Army in a Single Nanoparticle
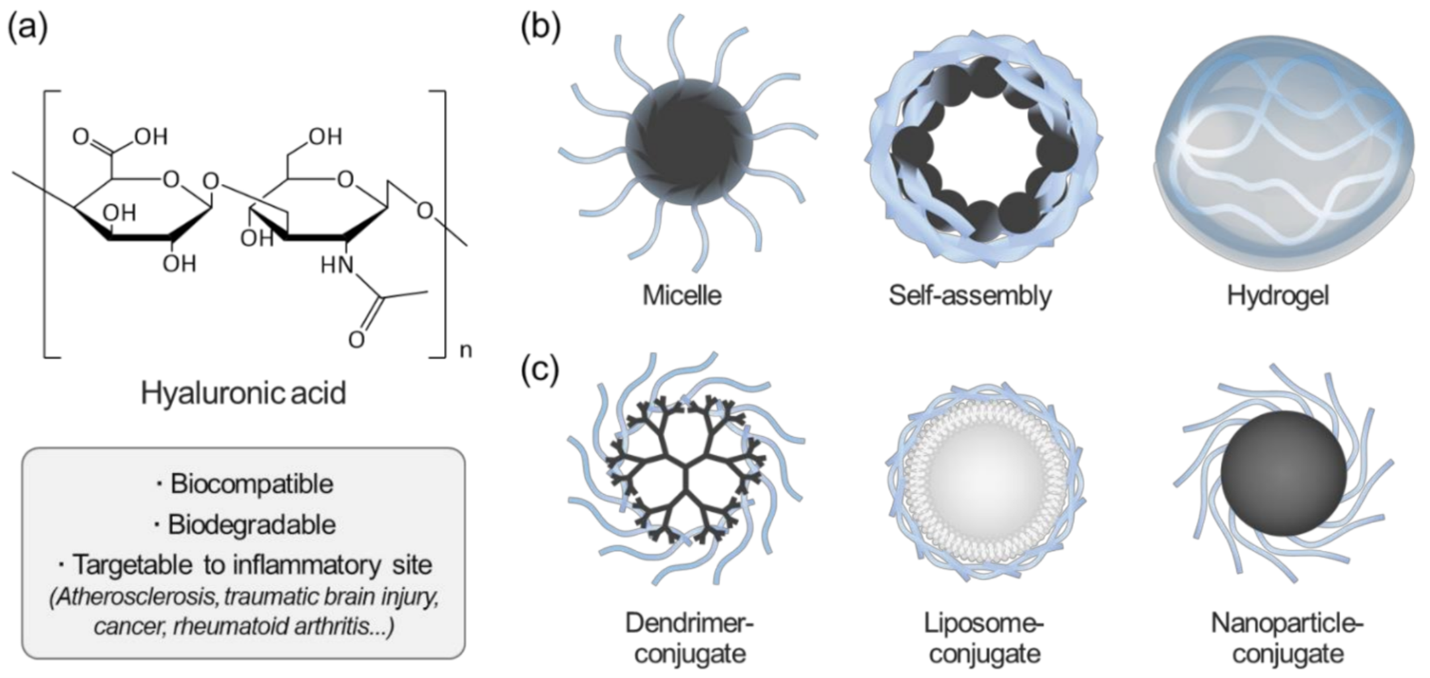
Overview of hyaluronic acid (HA)-based nanoparticles for biomedical applications. (a) Advantages of HA in biomedical applications; (b) Schematics of representative HA-based nanomaterials; (c) surface-modified nanomaterials using HA.
Rao, N.Vijayakameswara & Rho, Jun & Um, Wooram & Kumar, Epuri & Nguyen, Quy & Oh, Byeong & Kim, Wook & Park, Jae. (2020). Hyaluronic Acid Nanoparticles as Nanomedicine for Treatment of Inflammatory Diseases. Pharmaceutics. 12. 931. 10.3390/pharmaceutics12100931.
By adding immune checkpoint PD-L1 blocking peptides to the surface of the HANPs filled with Avasimibe, Yang’s team developed a new, multifunctional nanoparticle immune checkpoint therapy agent. The addition of another targeting peptide to urokinase plasminogen activator receptors (uPAR) further enhances targeted delivery of the nanoparticles to both tumor cells and atherosclerotic plaques. (Think of the receptors as an AirTag for the tumor that the HANP can track.)
Further, with about 200 PD-L1 blocking peptides on each nanoparticle, the new nanoparticle immunotherapy agent has a much stronger effect than an antibody-based immune checkpoint inhibitor, which is currently used to treat cancer patients. What’s more, the HANP has a greater capacity for carrying drugs, namely Avasimibe, so it can deliver higher dosages of the drug to elicit a stronger response from the tumor.
Two major benefits of HANPs are that they can:
- carry larger amounts of a drug to where it needs to go more effectively than the current treatments, and
- treat the cancer without a systemic side effect that can create or worsen cardiovascular diseases.
When filled with Avasimibe, the HANP even works to treat atherosclerosis while also treating the cancer. According to Yang, “There’s nothing like this treatment right now in either clinical trials or that’s commercially available. It’s one of the first of its kind.”
Next Steps
This treatment method is currently in pre-clinical studies with Yang and her team having worked on a dual disease mouse model. The results have been promising, giving the team an idea of how their drug delivery system will work to treat the concurrent diseases of cancer and atherosclerosis.
The team’s goal now is to get the HANP to Phase I clinical trials to continue their research and development. They are working on the publication of their research, and they have submitted a patent and several NIH grant applications with a focus on preclinical studies to obtain results required for the FDA approval of an Investigative New Drug application for a Phase I clinical trial in advanced colon cancer patients.

Lily Yang, MD, PhD, (right) and Lei Zhu, PhD, COO of MIGRA-Therapeutics and Assistant Professor in the Department of Surgery
Lily Yang
Challenging but Necessary Work
“We’ve had to be persistent,” Yang says. “We all know how challenging commercialization is. It’s like a valley of death. At each tiny step of development, we have to ask ourselves questions like ‘How do we give our nanoparticles a consistent shelf life?’ or ‘What is the best drug formulation?’ It can get tedious, but someone has to do it.”
With persistence and creativity, Yang and her team are fit for the job.
-- Jenna Woods
Tech ID: 22095
Read our technology brief.